Recent Studies in Laser-Surface Interactions
Our focus has been on the following previously unexplained problems:
- The desorption of 8-25 eV positive ions with very reproducible and sharp peaks (in energy) from 4-6 eV photon laser irradiation. We have extended this work down to 1.15 eV photons (1.06 µm wavelength) with startling results — we still see energetic ions.
- The origin of the apparent laser fluence threshold in ion desorption from these surfaces, and the highly non-linear dependence at higher fluences,
- The formation of fluorescing plumes under pulsed laser irradiation, i.e., how are the neutral ground state atoms excited into the high-lying excited states that yield atomic emissions.
- The broadening of the energy distributions of the desorbed ions — even production of ions moving backwards!
Our work continues to show the major role of surface and near surface defects generated by (a) mechanical processes (e.g., cleaving), (b) in some cases by the laser itself (via two photon processes), or (c) by irradiation with electrons. Our modeling efforts successfully fit the data for both ion energies, laser fluence dependence, and defect density dependence. Major papers have been published in Journal of Applied Physics and Appl. Phys. A.We have modeled the time and space evolution of weak, tenuous plasmas to explain (4) above.
Additional work:
- Discovered that electron damage, heating, and mechanical processes (abrasion) all dramatically strengthen UV laser interactions with single crystal CaHPO4 2H2O (a model biomaterial — bones, teeth) in ways that are similar to the effects observed on nitrates. The oxyanion appears to be the sensitive entity. Dehydration of the crystal is also very critical in that it generates considerable physical damage; we see evidence of accompanying defect production.
- Recently, we have discovered that during introduction of damage into crystals such as NaNO3 and CaHPO4.2H2O that we are producing structures at and/or near the surface that generate second harmonic light. For example, rhombohedral NaNO3 (Hanford Tank Matrix), is subjected to abrasion under a diamond stylus. We were attempting to stimulate the crystal with a Nd:YAG laser at 1064 nm (near IR – invisible to the eye–corresponding to photons with energies ~1.2 eV) directly behind the stylus (in the damage zone. What we discovered is that the crystal was emitting green light (corresponding to higher photon energies)–this light was seen by eye. After several tests we have found that the small damage zone is generating 2nd harmonic light; i.e., this region of the crystal is doubling the near IR laser light. The spectrum of the scattered light shown here is absolute proof of the doubling — a single green line at exactly 532 nm! Preliminary results on CaHPO4.2H2O another ionic single crystal material (also containing an oxyanion), is also showing doubling in the damage zone.
We hope to further understand the origin of this doubling and examine its implications. Our major interest includes determining the spatial extent of the source of doubling, the chemical nature of the source of doubling, and the relation it has to potential decomposition products — which may evolve from the surface during damage. The tools at our disposal are emission, absorption, and Raman spectroscopies, very high sensitivity, high resolution imaging, fast photon counting, imaging Auger and XPS, and mass spectroscopy (to look for volatile products). Although the crystals we are working on are model materials, It should be noted that phosphates are in several of the Hanford tanks, and second, brushite is a common biological material involved in precursors to bone growth, kidney stones, and build up of calcium deposits on teeth.
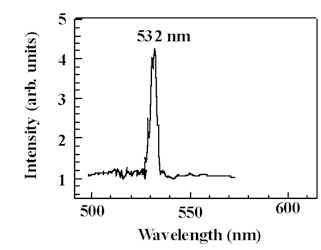
Our hypothesis for the case of NaNO3 is that the damage zone is transforming, due to partial decomposition of the nitrate ion, into a non-centrosymmetric crystallites (necessary condition for doubling). For example, KDP, the normal crystal used to double the Nd:YAG fundamental, has the broken symmetry. One candidate is orthorhombic NaNO2 (it has close to the same symmetry as KDP). We have tested for 2nd harmonic generation a nitrite single crystal we had grown earlier and found it to double almost as effectively as KDP. A similar mechanism may be involved in the damage zone of CaHPO4.2H2O.
- Laser Desorption
- Laser Ablation
- Chemical Analysis
- Surface Modification
- Thin Film Growth
- Materials: inorganic single crystals, glasses, polymers