Announcement: 2006 Gordon Conference on TRIBOLOGY (jtd Chair)
Tribology
Tribochemistry (mass spectroscopy; stressed enhanced dissolution)
Triboelectric effects (contract electrification; galvanic corrosion)
Energetics and damage/wear mechanisms
Fast time resolution measurements of wear
Plastic deformation processes during wear
EXPERIMENTAL TECHNIQUES:
Particle Detection (charged and neutral)
Mass Spectroscopy
Energy Analysis
Emission and Transmission Spectroscopy
Photoluminescence, Fracto-Emission, Chemi-Emission
AFM, STM, SEM, TEM
Transient Electrical Currents
Recent Studies in Tribology
(a) The use of AFM to study the combination of simultaneous tribological loading and corrosive chemical exposure. We have completed a major study on calcite where we find that mechanically stimulated corrosive attack requires full submersion of the specimen and tip into an aqueous medium is necessary. We made scanning force microscope (SFM) observations of enhanced calcite dissolution in aqueous solution due to mechanical stimulation induced by the SFM tip. Images and mechanical treatment were performed in saturated (_ 60 µM) CaCO3 solution adjusted to pH ~ 9. Small area scans of monolayer steps increase the step velocity in the scanned area (in the direction corresponding to dissolution) when the applied contact force is above a threshold value (about 160 nN for the tips employed). The step velocity could be increased at least an order of magnitude by scanning at even higher contact forces (e.g., 270 nN). This enhancement is a function of step orientation relative to the calcite lattice. Indentations near preexisting steps also locally enhance the step velocity. We have shown that the higher dissolution rates are caused by stress-induced increases in the rate of double-kink nucleation. We have quantified the wear rates due to linear scratches across steps in terms of the single atomic layers remove vs. normal force and tip velocity. A model has been developed that quantitatively fits our results very well, based on a simple stress dependent thermal activated process. The wear rate fits an equation of the form:
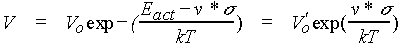 , (1)
, (1)
where Vo is the appropriate pre-exponential, Eact is the zero stress activation energy for double kink nucleation and v* is an activation volume. (Two such fits are shown for calcite in Fig. 2 below.) The stress applied by the SPM tip, s, was taken to be the peak radial, elastic stress at the boundary of contact for an infinitely stiff, spherical tip (a tensile stress).
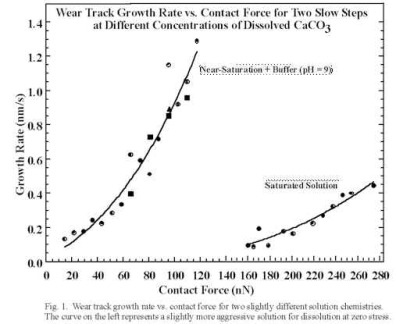
Fig. 2. Dissolution rates vs. Normal Force for Calcite; Two different solution chemistries.
Extension of this work to other slightly soluble crystals will allow us to test this model and examine the vulnerablity of various step structures (depending on crystallography) with considerable rigor. One example is brushite—monoclinic CaHPO4.2H2O—which is even less symmetric than calcite. Brushite forms plate-like crystals with two-dimensional layers of CaHPO4 separated by waters of hydration. Like calcite, brushite displays simple etch pits during dissolution. Unlike brushite, these pits (observed by SEM) are triangular and lack the symmetry of the underlying Bravais lattice. Two of the pit edges lie along lattice directions where the ion rows are not electrically neutral—counterintuitive to say the least! Periodic bonded chain analysis of the (010) surface show that the observed steps lie along particularly stable “double-bonded” chains; nevertheless, these chains do not necessarily display a zero dipole-moment perpendicular to the chain, as generally required in models of crystal growth and dissolution.
We have shown recently that brushite displays unit-cell deep etch pits during dissolution with orientations identical to the larger pits observed by SEM (edges along the [101], [201], and [001] directions). Further, the sharpest end of each triangular pit points in the same direction, even though two pit orientations can be constructed from the three observed edge directions. Therefore, the stability of a step depends on its “plus” or “minus” orientation—where we designate a plus step as one whose elevation increases when viewed facing the +[100] direction. In calcite, a similar asymmetry determines whether a step is “fast” or “slow.” In brushite, steps with unfavorable “signs” are simply not observed. Preliminary analysis suggests that the stability of +[001] steps is determined by the tilt of the phosphate groups with respect to the surface plane and the orientation of their hydrogen bonds. As we have shown, the brushite system is ideal for detailed testing of these issues in that anion geometry and bonding have such dramatic effects on step stability. A paper is almost completed that describes these results.
We plan on characterizing stress enhanced dissolution on (010) surfaces of single crystal brushite, measuring activation volumes and energies for kink nucleation on each of the three pit edges. By monitoring the evolution of wear tracks after formation, we will also determine the these parameters for kink propagation in both directions along each step. These results will be correlated with the molecular geometry of the corresponding kinks and steps. We will also vary the solution pH, which alters the chemical state of the anion (e.g., by forming HCO3-, H2PO42-) with little corresponding change in cation chemistry.
(b) The galvanic corrosive wear effects with perfluoropolyether lubricants.
Major Findings
_ New dynamic electrical techniques involving charge flow during interfacial traction provide details of the contact and adhesion of interacting surfaces (e.g., metal oxides, polymers, organic lubricants) with high time resolution. Electron transfer to certain organic lubricants may be an important lubrication degradation mechanism.
_ In certain metal/lubricant systems we have shown that galvanic potentials can develop and induce corrosive wear even when both substrates are initially identical. For the Al/Fomblin-Zdol system, the galvanic effects require surface modification as well as lubricant degradation. The charge transfer mechanisms and electrochemistry involved are under intense study.
We have devised small, sensitive devices to probe charge transfer between interacting surfaces in real-time. Charge transfer between conducting stylii and inorganic crystals and polymers have been successfully characterized. Lin et al. have shown that some perfluoropolyethers (PFPEs, e.g., Fomblin-Zdol—employed in computer hard disk drives) appear to be vulnerable to degradation by dissociative electron attachment in the presence of low energy electrons. Tribo-electron emission during wear is a potential source of such electrons. Recently, we have shown conclusively that electrons and positive ions are generated when an oxide surface is gently lifted from Zdol-coated surfaces in vacuo—without substrate damage. We are currently preparing our mass spectrometer to mass analyze these ions to determine if they are components of the oil. (We expect to see products of the form CxOyFz+, for x= 0,1,2, y = 0,1, and z = 1,2,3.) In addition to emission, we have also observed charge transfer between the departing oxide surface and the oil-coated substrate using transient current measurements. A study of very gentle make-break contact of a Al rider (with native oxide) and Fomblin-Zdol coated polymers (PMMA and PTFE—Teflon) showed conclusively that charge was transferred between the metal rider and the lubricant and that the oil captured electrons upon detachment. This route of charge exchange provides extremely low energy electrons to the Zdol which would be ideal for attachment processes, possibly including dissociative attachment.
In experiments designed to detect currents due to this charge separation, we unintentionally measured currents produced when an aluminum stylus was translated across a Fomblin-Zdol coated aluminum substrate. Anomalous potentials were observed that could not be attributed to contact electrification. Further study showed that these currents are instead due to galvanic reactions between the aluminum substrate and the PFPE lubricant. These galvanic currents were totally unexpected, because both “electrodes” in this system are nominally identical—i.e., we made a “battery” out of two pieces of Al. We showed that surface chemistry changes due to oxidation and reactions with the PFPE lubricant allow for galvanic potential differences of a few hundred mV. The implications are (a) this corrosive wear mechanism needs to be considered, and (b) these currents cannot be generated without lubricant degradation. The latter may be significant—especially since PFPE lubricants are often applied very sparingly in many applications (submicron down to ~monolayer films).